Energy of Adhesion
of Polymers
Wetting, spreading and adhesion phenomena are often described in terms of contact angles of liquids on a given surface. The figure below shows a solid surface (S) in contact with a droplet of liquids (L); we can distinguish between three different cases; if the liquid L fully wets the solid it tends to spread right over the surface. Thus, the contact angle θ is zero. In the second case the liquid does not spread over the surface but does wet it. The contact angle is between 0 and π/2. The third case, describes a liquid that does not wet the surface, i.e it tends to shrink away from the solid. For this situation, the contact angle is greater than π/2.
Wetting and Contact Angle
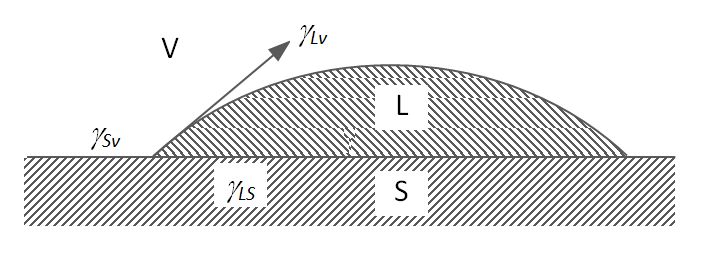
Equilibrium contact angles of liquids on solids are usually described with the Young’s equation:
γsv = γsl + γlv cos θ
γlv cos θ is called the adhesion tension. Complete wetting occurs when cos θ = 1 or θ = 0.
The energy of adhesion EA is the energy which is released when one liquid or solid surface comes in contact with another liquid or solid surface. During the wetting process, the two previously existing surfaces, which are in contact with vapor or air, disappear. As a result, energy is released due to the respective surface tensions σsv and σlv. At the same time, work is required to form the new interface which is the interfacial tension or energy σsl. Thus, the energy of adhesion can be described by the following equation:2
-EA = γlv + γsv - γsl
which is called the Dupre equation (1869).
energy of Adhesion
The energy of adhesion is usually negative since in most cases energy is released.
γlv + γsv > γsl
Obviously, adhesion between two materials is favored by a low interfacial free energy and high surface free energies of liquid and solid. Unfortunately, only λLV and θ can be directly measured. However, when we combine Dupre's equation with Young's equation, γlv and γsv can be eliminated:
-EA = γlv (1 + cos θ)
This equation is known as the Young-Dupre equation. For the special case that two identical materials come in contact, the contact angle is zero. Then the energy of adhesion becomes the energy of cohesion:
-EC = 2 · γlv
The energy of adhesion is a good measure for how easily a liquid wets another liquid or a solid. Its knowledge is important for processes such as coating, painting, cleaning etc. However, it is not a good measure for the bond forces present when a polymer adheres to a solid substrate, like a PSA adhering to a plastic. Polymer adhesion is a complex phenomenon and many other forces can be present which greatly increase the work of adhesion (peel force and work of separation).
Notes
True and accurate interfacial energies and contact angles of liquids at a polymer interface are excedingly difficult to obtain because a clean and smooth polymer surface is required which is difficult to maintain. Even a low energy surface will pick up contaminations from the environment during the manufacturing process and storage. If such contaminations are present, for example as a monolayer, it can act as a weak boundary layer and seriously affect the work of adhesion.
Here we assume a liquid in contact with a solid. However, the relations above equally apply to two liquids or two (semi) solid materials, like two viscoealstic polymers. A good example is a pressure sensitive adhesive tape in contact with a solid (plastic).